What Is Biomarker Testing?

ACMA
Oct 14, 2022
6 minutes read

Prior authorization for biomarker testing has been a topic of discussion due to California’s SB-535T legislation. Prior authorizations (PA or prior auth) require physicians and other healthcare providers (HCPs) to obtain approval from a health insurance company prior to providing specific services to patients. By requiring prior authorizations, health insurance companies prevent or delay life-saving tests, procedures, or medications.
As a result of California’s SB-535T legislation, insurance companies are not allowed to request prior authorization for biomarker testing for patients with advanced or metastatic cancer. On July 1st, 2022, the law became effective, making it one of the few states protecting patients’ rights to obtaining timely biomarker testing. Despite the law’s positive impact, biomarker testing still faces many challenges.
An American Cancer Society Cancer Action Network (ACS CAN) survey indicated lab turnaround times, costs, and inadequate insurance coverage create barriers for patients and providers fighting cancer. To diagnose and treat cancer, HCPs rely on biomarker testing. As medicine moves toward precision medicine and personalized care, biomarker testing is becoming increasingly critical.
According to Lisa Lacasse, president of ACS CAN, “…tremendous progress has been made in providing patients access to essential biomarker testing…it’s also important that such testing is readily available and is not delayed by coverage barriers.”
According to the ACS CAN 2021 survey of 315 oncology providers, lab turnaround time is the most challenging factor for biomarker testing. Out-of-pocket expenses are the second most common concern, followed by insurance coverage for biomarker tests. Based on the survey, 89% of providers agree that biomarker testing enables them to make better treatment recommendations, and 85% agree that enhancing access to biomarker testing will improve health equity.
As life science organizations propel precision medicine forward, more advanced cancer therapies are becoming available. In particular, biologics such as CAR-T cell therapy are becoming the standard of care for many cancers. More than half of the 62 cancer drugs introduced in the last five years require or recommend biomarker testing. Studies continue to reveal improved outcomes for patients treated with biomarker-based targeted cancer therapy.
With personalized care becoming more attainable, managed care pharmacists, prior authorization specialists, and field reimbursement specialists will increasingly face obstacles with biomarker test reimbursement. A better understanding of biomarker testing assists life sciences professionals and medical affairs teams in recognizing the value of biomarker tests to patients and the policy implications necessary to improve patient access. Presented here is a brief overview of biomarkers tests and their significance in medicine today and in the future.
What is a biomarker?
Biomarkers are biological indicators used to detect, diagnose, or monitor diseases.1,4 Detecting a biological indicator can range from a simple blood pressure measurement to whole genome sequencing (WGS). This article examines the use of biomarkers in cancer care, and why early and easy access to biomarker testing is vital to improving cancer survival rates.
Cancer biomarker testing is also known as:
- tumor testing
- tumor genetic testing
- genomic testing or genomic profiling
- molecular testing or molecular profiling
- somatic testing
- tumor subtyping
How are biomarkers used in cancer treatment?
Common cancer biomarkers include:
- PD-1/PDL-1: An immune response-regulating protein on T cells.
- HER2 (ERBB2): gene used to identify non-small cell lung cancer (NSCLC) and breast cancer.
- KRAS/NRAS: gene used to identify colorectal cancer.
- NTRK gene fusion: used to assess solid tumors.
- Circulating tumor cells of epithelial origin (CELLSEARCH): identify metastatic breast, prostate, and colorectal cancers.
Biomarkers are used to predict future cancer risks, cancer progression, and response to therapy. Some biomarkers are even used as surrogate endpoints in clinical trials but are not a substitute for clinical endpoints. Biomarker testing is crucial to collecting real-world evidence (RWE) since the results can provide a more precise picture of an individual’s health.
In the past, patients with various cancers received similar therapy, but research has shown that tumors have unique features. In recent years, doctors have increasingly used cancer biomarkers to gain additional insight into a patient’s tumor and determine the most effective treatment for their unique cancer. For example, detecting elevated levels of a specific biomarker can guide a treatment plan toward a more precise therapy.
Cancer cells produce biomarker profiles specific to each tumor. Biomarker tests for cancer include checking for alterations in DNA sequences and measuring RNA or protein levels. Many biomarker tests rely on next-generation sequencing (NGS), which identifies nucleotide sequences in whole genomes or targeted regions. NGS has made identifying potential biomarkers easier and more cost-effective.
The search for biomarkers is ongoing in many areas of cancer research.3 Aside from identifying and determining new biomarkers, current biomarker research focuses on the following areas:
1. Immunotherapy response: detect immune response biomarkers, enabling patients to receive tailored immunotherapies.
2. Liquid biopsies: monitor the tumor response to specific therapies.
3. Minimal residual disease: estimates remaining disease through blood testing.
4. Pharmacodynamic markers: determine if a specific cancer therapy is effective.
What are the types of biomarkers?
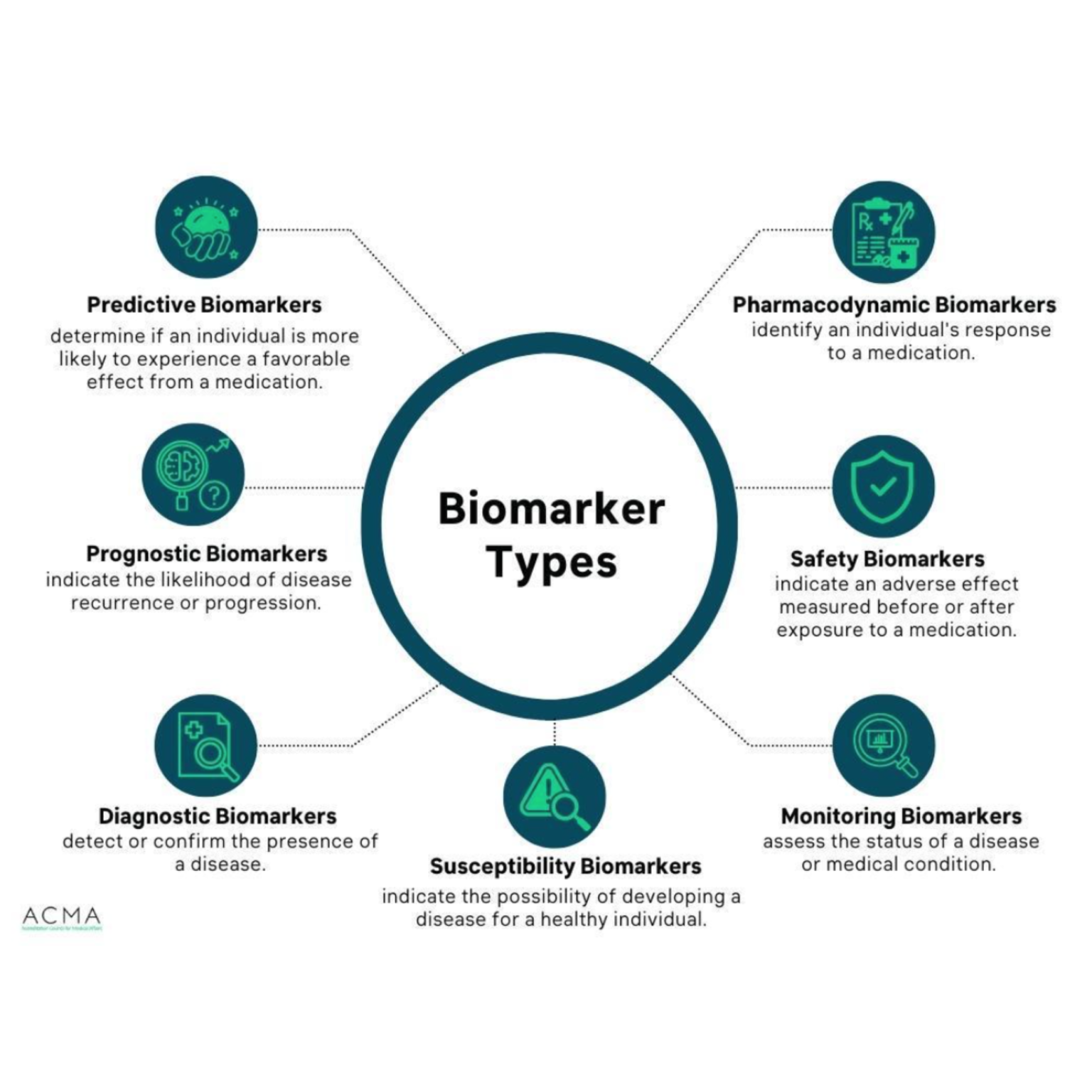
The FDA categorizes biomarkers into seven distinct types. A biomarker, however, can belong to more than one category. Figure 1 illustrates the types of biomarkers.
Does insurance cover biomarker testing?
Payers often determine coverage based on the type and clinical utility of biomarkers. While insurance typically pays for diagnostic and prognostic biomarkers, insurance is less likely to cover predictive biomarkers if they lack sufficient clinical evidence supporting their value. Clinical utility refers to a test’s likelihood to initiate intervention and improve health outcomes.

Private and public insurance may also provide varying degrees of coverage depending on the type of cancer. Private payers cover solid tumor biomarkers, but liquid biomarkers are less commonly covered. Biomarker testing is considered the standard of care for detecting solid tumors due to their documented clinical utility by clinical trials and real-world evidence. However, payers remain skeptical about the clinical utility of tests for large panels of biomarkers.

The same ACS CAN 2021 survey asked providers what challenges they faced in biomarker testing and what solutions they might offer. Here are a few of the providers’ answers:
- “I think the current situation is that the budget for reimbursing test expenses is somewhat insufficient.”
- “Insurance providers need a better understanding of the literature justifying using biomarkers.”
- “Insurance coverage is formidable.”
- “Better provider education necessary.”
As part of an American Society for Radiation Oncology Task Force report, possible solutions were proposed to ensure all stakeholders in managed care understand the clinical utility of biomarker testing. Among the possible solutions were:2
- Ensure all stakeholders adhere to the same evidentiary standards in clinical utility, based on evidence generated both within and outside of clinical trials
- Improve the coordination and transparency of the federal regulatory process for biomarker testing
- Assess the clinical utility of biomarker tests for molecularly targeted therapies on an ongoing basis
- Promote equity in access to biomarker tests
Health equity is only achieved when biomarker testing is widely accessible and covered by public and private insurance. If additional laws similar to California’s recent legislation pass, the U.S. healthcare system will improve and ensure cancer patients receive optimal care. Learn more about biomarkers and prior authorizations by visiting the following resources.
Biomarker Resources
- Read the BEST (Biomarkers, EndpointS, and other Tools) Resource from the FDA-NIH Biomarker Working Group.
- Listen to Dr. Janet Woodcock, FDA’s Principal Deputy Commissioner, discuss how biomarkers influence drug development.
- Access the Center for Medicare and Medicaid Services’ Billing and Coding: Biomarkers for Oncology resource.
- Learn about the pharmacist’s role in prior authorization.
- Become an expert in immunotherapies and other biologics through the ACMA’s Board Certification in Biologics and Biosimilars.
References
1. Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). 2018;243(3):213-221. doi:10.1177/1535370217750088.
2. Graig LA, Phillips JK, Moses HL, et al. Erratum to: Wallner PE, Anscher MS, Barker CA, et al. Current status and recommendations for the future of research, teaching, and testing in the biological sciences of radiation oncology: report of the American Society for Radiation Oncology Cancer Biology/Radiation Biology Task Force, executive summary. Int J Radiat Oncol Biol Phys 2014;88:11-17. Int J Radiat Oncol Biol Phys. 2015;91(5):1115. doi:10.1016/j.ijrobp.2014.12.031.
3. Hodgson DR, Whittaker RD, Herath A, Amakye D, Clack G. Biomarkers in oncology drug development. Molecular Oncology. 2009;3(1):24-32. doi:10.1016/j.molonc.2008.12.002.
4. Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463-466. doi:10.1097/COH.0b013e32833ed177.

Become excellent in prior authorization, reimbursement & market access: Become a prior authorization certified specialist
PACS Links
What is PACS?
PACS reviews
Why certify?
PACS price
PACS recertification
PACS extension
PACS FAQs
Quick contact
info@acmainfo.org